Clinical pharmacology of targeted therapy in oncology
Regina Demlová
Subchapter list
13.4 Principles of classification of targeted drugs
It is not easy to present a precise classification of targeted drugs because the didactic classification of these drugs has not been established yet. The most straightforward classification is based on the target action of each drug, and in general, the mechanisms of action can be divided into several groups below. Indeed, other schematic classifications may be encountered, as the drugs listed often overlap in many ways; moreover, the signaling pathways for a given malignancy may not exclusively fulfill a pathological function in that tumor but may also inhibit other oncoproteins in a "crossover" manner. The aim of the text is not to give a complete overview of targeted drugs; it will be presented in more detail in the lecture. An overview of the current indications and TKIs is found in Table 13.2.
Table 13.2: Overview of selected TKIs used in oncology
| Drug | Target | Indication |
| afatinib | EFGR, HER2 | NSCLC |
| bevacizumab | VEGF | NSCLC, ovarian Ca, RCC, colorectal cancer, breast cancer |
| cetuximab | EFGR | Colorectal Ca and head and neck Ca |
| crizotinib | MET, ALK | NSCLC |
| dabrafenib | BRAF | Melanoma, NSCLC |
| erlotinib | EGFR | NSCLC, pancreatic Ca |
| imatinib | BCR-ABL, KIT, PDGFR, SRC | CML, ALL, GIST |
| lapatinib | EGFR and HER2 | Breast Ca |
| palbociclib | CDK-4/6, | Breast Ca |
| pazopanib | VEGFR | RCC, Sarcoma |
| regorafenib | VEGFR, TIE2, KIT, RET, RAF-1, BRAF, BRAFV600, PDGFR, FGFR | Colon Ca, GIST, HCC |
| ruxolitinib | JAK | Myelofibrosis |
| sorafenib | BRAF, Flt-3, RET, KIT, VEGFR, PDGFR-β | HCC |
| sunitinib | VEGFR, KIT, RET, PDGFR | GIST, pNET, RCC |
| trametinib | MEK | Melanoma, NSCLC |
| trastuzumab | HER2 | Breast Ca, gastric cancer |
ALL – Acute lymphoblastic leukemia, Ca – cancer, CML – chronic myeloid leukemia, GIST – gastrointestinal stromal tumor, HCC – hepatocellular carcinoma, NSCLC – non-small cell lung cancer, pNET – pancreatic neuroendocrine tumor, RCC – renal cell carcinoma
13.4.1 Inhibition of endogenous target receptor ligand
This is the mechanism of action of agents that can bind and thereby neutralize an endogenous ligand that, upon binding to a specific membrane receptor, triggers a sequence of intracellular processes referred to as a transduction cascade (e.g., bevacizumab as a VEGF inhibitor) (Figure 13.1).
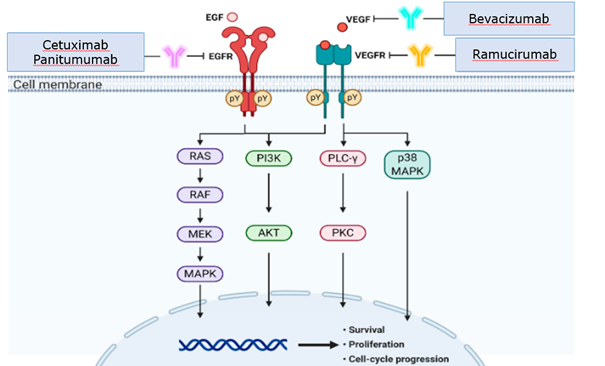
13.4.2 Inhibition of growth factor receptors
Targeted drugs acting in this way can inhibit growth factor receptors. Pharmacologically, they may be monoclonal antibodies that show an affinity for the extracellular domain of a given receptor while acting as its antagonist. Many monoclonal antibodies operate this way (e.g., cetuximab as an EGFR inhibitor). The second option is to inhibit the intracellular domain of the receptor. This is how small chemically defined tyrosine kinase receptor inhibitors work (e.g., erlotinib as an EGFR inhibitor). (Figure 13.2). They are indicated for treating tumors with high activity of the relevant growth factor receptors, routinely determined in advance in precision oncology principles.
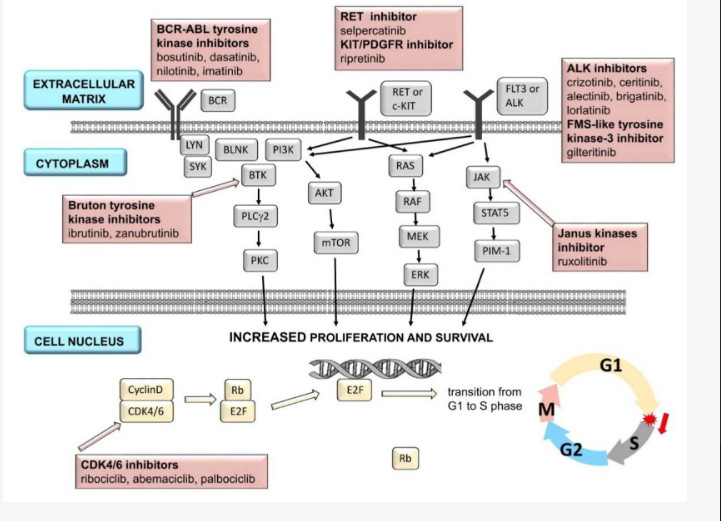
13.4.3 Inhibition of intracellular tumor cell signaling
This group is undergoing very intensive development, and many drugs have been introduced into clinical practice that interfere with some sites of the intracellular downstream signaling pathway. Each signaling molecule has its own specific (protein) kinase. Some drugs inhibit only one type of kinase (selective inhibitors) (e.g., trametinib – MEK inhibitor); others (non-selective) inhibit several types of kinases simultaneously (e.g., sorafenib – BRAF, Flt-3, RET, KIT, VEGFR, PDGFR-β).
13.4.4 Inhibition of cell cycle regulatory proteins
The cell cycle is a very complex process that can be influenced by several strategies. One promising therapeutic direction is the development of small molecules that inhibit cyclin-dependent kinase (CDK) activity by interacting with the binding site for ATP. Cyclin-dependent kinases are among the major regulators of the cell cycle. They are serine-threonine kinases responsible for phosphorylation and associated activation of cyclins. The key one is cyclin D, which is activated by the phosphorylated form of CDK 4/6. Activation of cyclin D results in phosphorylation of the retinoblastoma (Rb) protein, which inactivates the transcription factor E2F. This process allows the cell to pass through the G1 checkpoint from the G to S phase of the cell cycle. Apoptosis is activated if the cell fails to complete the above processes and the cell cycle does not proceed to the S phase. Alteration of the above-described mechanism leads to loss of cell cycle regulation and the development of hormone resistance. Increased expression and activity of CDK 4/6 are frequent in breast cancer cells, and several gene mutations leading to increased cyclin D production are also present. Examples of CDK 4/6 inhibitors used in clinical practice are palbociclib, ribociclib, or abemaciclib.
13.4.5 Example of pharmacological influence on EGFR
One target receptor of interest early in developing targeted drugs is the epidermal growth factor receptor (EGFR). In this example, we will attempt to demonstrate the options for pharmacologically influencing the receptor with targeted drugs that act by some of the mechanisms of action described above. The EGFR family includes 4 receptor subtypes, EGFR, also known as ErbB-1 or HER1, HER2/Neu (ErbB-2), ErbB-3/HER3 and ErbB-4/HER4. Modifications in the EGF receptor family often accompany the occurrence and development of several types of carcinomas and fundamentally interfere with the carcinogenesis process. The epidermal growth factor receptor is present in some carcinomas and contributes significantly to their development. For this reason, the epidermal growth factor receptor has been selected as one of the first targets for modern drugs.
Application exercises:
One of the EGF receptor subtypes is HER2/Neu (ErbB-2). What targeted drugs are you aware of that affect it, and what are their most common adverse effects?
-
Answer, solution
The HER2/Neu receptor can be affected pharmacologically by administering a specific monoclonal antibody inhibiting the receptor's extracellular domain or an inhibitor of its intracellular kinase domain. Trastuzumab is a monoclonal antibody against HER-2/neu, and a prerequisite for successful treatment with trastuzumab is the accurate and reliable determination of HER-2 proteine expression or gene amplification in the tumor. It occurs in 25–30% of women with breast cancer and corresponds with a poor prognosis of the disease. According to clinical trials, trastuzumab is effective in monotherapy and combination with chemotherapy in patients with early or metastatic breast cancer and increased HER-2/neu expression and in metastatic gastric cancer adenocarcinoma. Treatment with trastuzumab is usually well tolerated; cardiotoxicity may be a more serious late adverse effect. Other HER2/Neu-affecting mABs include pertuzumab and trastuzumab emtansine and trastuzumab deruxtecan. The two latter are conjugates of trastuzumab and the cytostatic agent emtansine and deruxtecan, respectively – the antibody here provides targeted delivery of an otherwise highly toxic cytostatic agent. Trastuzumab deruxtecan recently showed to provide benefit even with lower HER2/Neu expression levels compared to traditional criteria.
Another therapeutic option to affect HER2/Neu is inhibitors blocking the tyrosine kinase activity of this receptor. Small molecules of these active agents bind competitively to the binding site for the macroergic phosphate ATP on the receptor's intracellular domain. This blocks phosphorylation and signal transduction. Lapatinib is a dual inhibitor – it blocks the ATP-binding site of tyrosine kinases associated with both EGFR and Her2/neu, preventing autophosphorylation and signal initiation. It is indicated for Her2/neu therapy in breast cancer. The most common adverse side effects are diarrhea and skin toxicity.
Would it be possible to combine trastuzumab with lapatinib to increase the anti-tumor effect? Justify.
-
Answer, solution
Yes, the combination of lapatinib and trastuzumab significantly improved pathological complete response (pCR), event-free survival (EFS), and overall survival (OS) in HER2-positive breast cancer compared with trastuzumab or lapatinib alone because they have complementary mechanisms of action and synergistic anti-tumor activity in HER2-positive breast cancer.
Application exercises:
Similarly, another subtype is the EGFR1 receptor, also known as ErbB-1 or HER1. What targeted drugs do you know that affect it?
-
Answer, solution
Cetuximab is a chimeric monoclonal antibody that binds competitively to the extracellular domain of EGFR (HER1), thereby antagonizing the binding of other potential activators. Clinical trials with cetuximab have shown very good tolerability without hematological toxicity. Currently, cetuximab is mainly used in combination with chemotherapy regimens in metastatic colorectal cancer (see above). The humanized monoclonal antibody panitumumab has a similar mechanism of action.
Gefitinib is a small molecule, quinazoline derivative, inhibiting EGFR (HER1) tyrosine kinase activity with good bioavailability after oral administration. It is mainly used in patients with advanced NSCLC progressing after chemotherapy failure. Gefitinib has demonstrated very good tolerability without hematological toxicity. A similar agent is erlotinib, which has been shown to be effective in ovarian, head and neck, and non-small cell lung cancer. Adverse effects are similar to gefitinib, diarrhea, and skin toxicity.
What adverse effects might be expected when applying gefitinib or cetuximab in clinical practice?
-
Answer, solution
Given the function of the epidermal growth factor receptor to regulate the physiological epidermal development, skin adverse effects – such as acne, rash, dry skin, paronychia – are described for both products.